Abstract
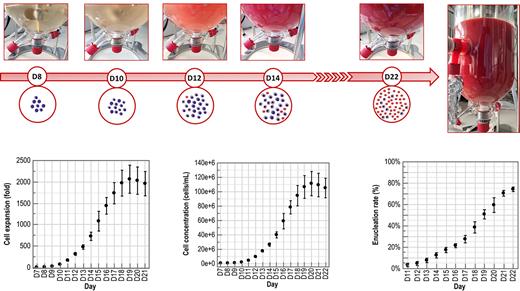
The ex vivo generation of cultured red blood cells (cRBC) from stem cells has been one of the most exciting lines of research for more than 20 years, with the ultimate goal of improving the standard of care for anemic patients. In particular, cRBCs are expected to benefit to transfusion-dependent patients by reducing the transfusion frequency, thanks to an extended lifespan in circulation. A few studies have already been able to demonstrate the quality of the product in animals and even in humans. However, while some teams have focused on industrial production issues, no convincing solutions for offering cRBCs as an industrial product for transfusion is currently available. Despite the medical promise of this approach, the deployment of this new biological therapy is still hampered by three technological obstacles. (i) With state-of-the-art serial-batch culture process, the production of a single unit of cRBCs (approximately 1.4x1012 cells) would require more than 150 m² in 2D culture, or three >450-L bioreactors, both unrealistic options. (ii) Production costs are prohibitive: we estimate that it would cost more than 350,000 euros in raw materials alone to produce one unit of cRBCs using standard processes. (iii) Finally, the challenge of final cRBC purification has never been broached.
Several technological breakthroughs are therefore needed to make this concept accessible. We embraced these challenges by bringing together expertises from process engineering and fundamental biology and developed a new perfusion concept (Ultrafiltration Plus PERfusion CULTure - UPPERCULT), which overcomes the first two obstacles, demultiplying volumetric productivity and dividing production costs by a factor of 29. Specifically, UPPERCULT circumvents the massive protein waste that occurs during standard microfiltration-based perfusion, where the whole medium is continuously eliminated in the permeate, including expensive proteins. The latter, such as transferrin, albumin and growth factors usually account for over 95% of medium cost. By using ultrafiltration membranes that retain proteins within the bioreactor and thanks to fine analysis of individual protein needs and transferrin recycling, UPPERCULT technology is able to reduce the necessary protein quantities from 90 to 98%, which implies a sharp reduction in production costs. By combining this process with negotiated raw materials purchase prices for industrial scale, production costs are expected to be less than 1,500 euros/cRBC unit in realistic scale facilities. We also developed a complete downstream process which eliminates from 90 to 99.5% of cellular and molecular impurities with less than 25% product loss and effectively removes the third obstacle.
From the essential point of view of the functionality of cRBCs, in terms of morphological and biochemical characteristics, it should first be noted that our cRBCs are almost identical to those made from traditional static cultures, which already demonstrated their potential in the past (Giarratana et al., Blood, 2011). Moreover, their functional evaluation to pass through the smallest constrictions of the microcirculation is excellent, similar to that of native RBCs, demonstrating their high deformability. These results support the safety of future cRBC-based products.
With an overall global productivity of 153 ± 58 cRBC units per adult hematopoietic stem cell donation, our results offer a credible pathway towards the industrial production of cRBCs for transfusion.
Disclosures
Rousseau:Erypharm: Current Employment. Rasschaert:Erypharm: Ended employment in the past 24 months. Maestrali:Erypharm: Ended employment in the past 24 months. Lardier:Erypharm: Ended employment in the past 24 months. Mathieu:Erypharm: Ended employment in the past 24 months. Giarratana:Erypharm: Ended employment in the past 24 months. Loche:Erypharm: Ended employment in the past 24 months. Mazurier:Erypharm: Ended employment in the past 24 months. Le Mignot:Erypharm: Ended employment in the past 24 months. Houx:Erypharm: Ended employment in the past 24 months. Douay:Erypharm: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal